News
New insights into the catalytic mechanism of an ABC membrane transporter
November 2018. The transport of substrates across cell membranes is vital to all living organisms. Cells have different types of transporter proteins integrated into their membranes that provide transmembrane pathways for a wide variety of substrates, ranging from ions to macromolecules. Of particular importance are the ubiquitous ATP binding cassette transporters, also known as ABC transporters. Using advanced spectroscopic methods, a team of scientists led by Clemens Glaubitz from Goethe University Frankfurt provides important new insights into how ABC transporters function.
The study focused on the MsbA transporter, an ABC exporter that transports lipopolysaccharides across cell membranes. ABC transporters have nucleotide-binding domains that changed relatively little during the course of evolution. The hydrolysis of ATP catalyzed by these domains is the key event for the functional mechanism of ABC proteins. Surprisingly, the MsbA transporter is in addition able to catalyze a reverse adenylate kinase-like reaction resulting in ADP molecules being converted to ATP and AMP, as the team recently demonstrated. This coupled ATPase-rAK activity could be of importance when levels of ATP are depleted in a cell. Both reactions are mediated by the same conserved nucleotide-binding domains.
The team set out to study the structural foundations underlying the nucleotide binding. Several 3D structures of the MsBA transporter had been published, which provided important information about how lipopolysaccharide transport may take place. However, there were some puzzling differences between these structures, which the present study set forth to further investigate with a combination of methods.
Spectroscopic approaches play an important role in bridging the gap between biochemical and structural data. The team employed a combination of conventional- and DNP-enhanced solid-state nuclear magnetic resonance spectroscopy (NMR), as well as pulsed-EPR (with the Prisner lab, Frankfurt) and also applied MD simulations (with the Soares lab, Lisbon) to aid data interpretation. Beforehand, the MsbA transporter protein was reconstituted into lipid bilayers and trapped in a range of catalytic states.
The results provide evidence for an additional nucleotide-binding site. And DNP-mediated signal enhancement enabled, for the first time, the spectroscopic identification of nucleotide-protein contacts. The study highlights the capability of DNP-mediated NMR to explore stable conformations as well as intermediate states that might be difficult to capture by electron microscopy or crystallization techniques.
One further important aspect of nucleotide-protein interactions is the coordination of the required metal ion, which was addressed in this study by Mn-EPR spectroscopy. Mn-EPR, especially at high field, is a rather attractive option for metal-binding proteins such as ABC transporters since mechanistic insight could be obtained without mutations or spin labeling. Here, the first application of relaxation-induced dipolar modulation enhancement (RIDME) to an ABC transporter demonstrates that dipolar couplings can be obtained with high sensitivity directly within liposomes. This will have implications for future studies on conformational changes of membrane proteins by EPR.
This study emphasizes the importance of advanced spectroscopic approaches for obtaining insight into the catalytic mechanism of membrane proteins directly within the lipid bilayer. The description of novel nucleotide binding modes extends the paradigm of how ABC transporters appear to work, and the impact may be even broader if it applies to the ABC superfamily as a whole.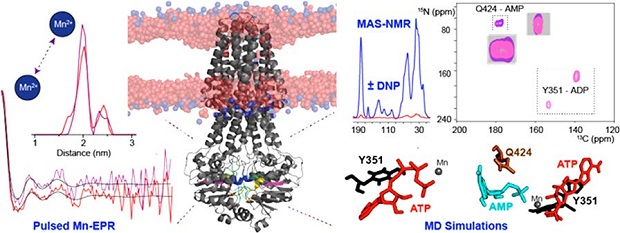
Contact:
Clemens Glaubitz, Center for Biomolecular Magnetic Resonance (BMRZ) and Institute of Biophysical Chemistry, Riedberg Campus, Goethe University, Frankfurt/Main, Germany, glaubitz@em.uni-frankfurt.de
Publication:
Kaur H, Abreu B, Akhmetzyanov D, Lakatos-Karoly A, Soares CM, Prisner T, Glaubitz C (2018) Unexplored nucleotide binding modes for the ABC exporter MsbA. Journal of the American Chemical Society 140: 14112–14125. http://dx.doi.org/10.1021/jacs.8b06739
Cluster of Excellence Macromolecular Complexes, Frankfurt am Main, Germany

