News
Structural insights into ion conduction by channelrhodopsin 2
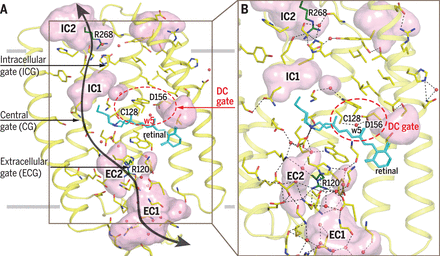
November 2017. Channelrhodopsins are membrane channel proteins whose gating is controlled by light. In their native setting, they allow green algae to move in response to light. Their expression in neurons allows precise control of neural activity, an approach known as optogenetics. A team of scientists led by Valentin Gordeliy from the Research Centre Juelich and Ernst Bamberg from the Max Planck Institute of Biophysics in Frankfurt have solved the structure of channelrhodopsin 2, the most widely used optogenetics tool, as well as the structure of a mutant with a longer open-state lifetime. Light activation perturbs an intricate hydrogen-bonding network to open the channel. The structures provide a basis for designing better optogenetic tools.
Ion channels are integral membrane proteins that upon stimulation modulate the flow of ions across the cell or organelle membrane. The resulting electrical signals are involved in biological functions such as electrochemical transmission and information processing in neurons. Channelrhodopsins (ChRs) appear to be unusual channels. They belong to the large family of microbial rhodopsins, seven-helical transmembrane proteins containing retinal as chromophore. Photon absorption initiates retinal isomerization resulting in a photocycle, with different spectroscopically distinguishable intermediates, thereby controlling the opening and closing of the channel. In 2003, it was demonstrated that light-induced currents by heterologously expressed ChR2 can be used to change a host’s membrane potential. The concept was further applied to precisely control muscle and neural activity by using light-induced depolarization to trigger an action potential in neurons expressing ChR2. This optogenetic approach with ChR2 and other ChRs has been widely used for remote control of neural cells in culture and in living animals with high spatiotemporal resolution. It is also used in biomedical studies aimed to cure severe diseases.
Despite the wealth of biochemical and biophysical data, a high-resolution structure and structural mechanisms of a native ChR2 were up to now not known. Given that ChR2 is the most frequently used tool in optogenetics, knowing its structure is of high importance. Deciphering the structure of the native channel is shedding light on how the light-induced changes at the retinal Schiff base (RSB) are linked to the channel operation and may make engineering of enhanced optogenetic tools more efficient. The determined structures of ChR2 and its C128T mutant present the molecular basis for the understanding of ChR functioning. They provide insights into mechanisms of channel opening and closing. More ...
Contact:
Ernst Bamberg, Max Planck Institute of Biophysics, Frankfurt am Main, Germany, ernst.bamberg(at)biophys.mpg.de
Publication:
Volkov O, Kovalev K, Polovinkin V, Borshchevskiy V, Bamann C, Astashkin R, Marin E, Popov A, Balandin T, Willbold D, Buldt G, Bamberg E*, Gordeliy V* (2017) Structural insights into ion conduction by channelrhodopsin 2. Science 358: published online 24 November 2017. http://dx.doi.org/10.1126/science.aan8862
Cluster of Excellence Macromolecular Complexes, Frankfurt am Main, Germany