News
Understanding and modulating partial FXR activation
July 2019. Modulation of the nuclear receptor protein FXR is a promising strategy to treat several severe metabolic diseases. Pharmacological administration of full FXR agonists has been plagued by mechanism-based side effects. A team of scientists from Frankfurt, Heidelberg and Gothenburg now reports a modulator that partially activates FXR. Through crystallographic and NMR-based structural biology studies the team unravelled the structure of human FXR in complex with an agonist and deciphered the molecular mechanism that drives partial FXR activation.
The bile acid-sensing transcription factor farnesoid X receptor (FXR) regulates multiple metabolic processes. This nuclear receptor protein has received considerable interest in relation to drug discovery and pharmacology due to its important role in metabolism and its value as a drug target to treat liver disorders and metabolic diseases.
One FXR targeting drug, called OCA, has already received market approval and confirmed the therapeutic value of targeting this rezeptor protein. However, as FXR regulates multiple metabolic processes and participates in various endocrine functions, FXR modulation is also prone to cause mechanism-based side-effects. Clinical trials of OCA have already shown that extensive FXR activation disrupts cholesterol homoeostasis with FXR activation blocking metabolic conversion of cholesterol to bile acids. Its long-term pharmacological blockade may have serious consequences.
Partial activation appears to be an avenue to safely exploit FXR as a drug target as such an approach could reduce side effects such as the loss of metabolic cholesterol degradation. Similar strategies have been proposed for other nuclear receptor proteins that are known to cause unwanted sideeffects. However, a better understanding of partial FXR activation on molecular level is necessary to explore such a strategy.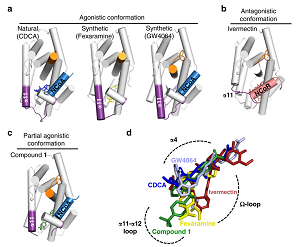 A team of scientists from Frankfurt, Heidelberg and Gothenburg therefore joined forces to study the structural determinants of partial FXR agonism by solving co-crystal structures and investigating the conformational dynamics of quaternary complexes involving the FXR-LBD, co-activator and co-repressor peptides as well as a variety of FXR ligands by NMR spectroscopy.
A team of scientists from Frankfurt, Heidelberg and Gothenburg therefore joined forces to study the structural determinants of partial FXR agonism by solving co-crystal structures and investigating the conformational dynamics of quaternary complexes involving the FXR-LBD, co-activator and co-repressor peptides as well as a variety of FXR ligands by NMR spectroscopy.
The scientists report in the journal Nature Communications the elucidation of the molecular mechanism that drives partial FXR activation. Natural and synthetic FXR agonists stabilize formation of an extended helix α11 and the α11-α12 loop upon binding. This strengthens a network of hydrogen bonds, repositions helix α12 and enables co-activator recruitment. Partial agonism in contrast is conferred by a kink in helix α11 that destabilizes the α11-α12 loop, a critical determinant for helix α12 orientation. Thereby, the synthetic partial agonist induces conformational states, capable of recruiting both co-repressors and co-activators leading to an equilibrium of co-activator and co-repressor binding. More...
These results may significantly aid future drug discovery targeting nuclear receptor proteins and the development of safer FXR modulating medication.
Contacts:
Harald Schwalbe (Center for Biomolecular Magnetic Resonance (BMRZ), Institute for Organic Chemistry & Chemical Biology, schwalbe@nmr.uni-frankfurt.de) and Daniel Merk (Institute of Pharmaceutical Chemistry, merk@pharmchem.uni-frankfurt.de), Goethe University, Frankfurt/Main, Germany
Publication:
Merk D*, Sreeramulu S, Kudlinzki D, Saxena K, Linhard V, Gande SL, Hiller F, Lamers C, Nilsson E, Aagaard A, Wissler L, Dekker N, Bamberg K, Schubert-Zsilavecz M, Schwalbe H* (2019) Molecular tuning of farnesoid X receptor partial agonism. Nature Communications 10: 2915. http://dx.doi.org/10.1038/s41467-019-10853-2

