News Archive
New Nature paper by Volker Dötsch and colleagues
Structural basis for the selectivity of the external thioesterase of the surfactin synthetase
The group of Volker Dötsch published their latest findings on 14 August 2008 in Nature. The research was conducted in collaboration with scientists from Marburg, Massachusetts and Russia [link to full paper].
See also coverage in Nature News and Views
Press release [in German]
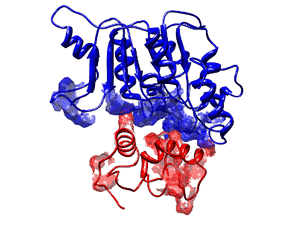
Figure: Structure of the thioesterase II in complex with a peptidyl carrier protein (PCP). Both proteins are essential components of Non-ribosomal peptide synthetases. These megaenzyme complexes produce antibiotics as well as many other pharmaceutically relevant molecules. The PCPs are responsible for moving the growing peptide chain between the individual reaction centers while the thioesterase II repairs PCPs that have been non-enzymatically modified on their phosphopantetheinyl cofactor. Without this repair service of the thioesterase the production of Non-ribosomal peptide synthetases would be reduced by more than 80%.

