News Archive
Dimers of ATP synthase self-assemble into rows and induce membrane curvature
February 2019. ATP synthases are dynamic membrane protein complexes, which play a crucial role in cellular bioenergetics as they produce ATP, the cell’s universal energy currency. Mitochondrial ATP synthases have been a long-standing research interest of scientists at the Max Planck Institute of Biophysics in Frankfurt. A team led by Werner Kühlbrandt now reports that mitochondrial ATP synthase dimers assemble spontaneously into rows upon membrane reconstitution and that these rows bend the membrane of the mitochondria.
Mitochondria are essential cell organelles that play a central role in bioenergetics and cell physiology. Like their bacterial ancestors, mitochondria have an outer and an inner membrane. The inner membrane is folded into deep membrane invaginations called cristae. The cristae increase the inner membrane surface to accommodate large numbers of respiratory chain complexes and ATP synthase.
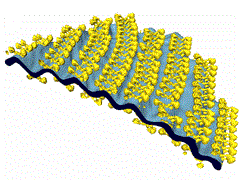 Mitochondrial ATP synthases form dimers that consist of two identical monomers of ATP synthase. Computer simulations suggested that the dimer rows bend the membrane locally, but this had not been shown experimentally. In their new study, the researchers used electron cryotomography to provide the experimental proof. They found that ATP synthase dimers assemble spontaneously into rows upon membrane reconstitution, and that these rows bend the membrane. The assembly of ATP synthase dimers into rows is most likely the first step in the formation of mitochondrial cristae. Their report was recently published in Proceedings of the National Academy of Sciences of the USA.
Mitochondrial ATP synthases form dimers that consist of two identical monomers of ATP synthase. Computer simulations suggested that the dimer rows bend the membrane locally, but this had not been shown experimentally. In their new study, the researchers used electron cryotomography to provide the experimental proof. They found that ATP synthase dimers assemble spontaneously into rows upon membrane reconstitution, and that these rows bend the membrane. The assembly of ATP synthase dimers into rows is most likely the first step in the formation of mitochondrial cristae. Their report was recently published in Proceedings of the National Academy of Sciences of the USA.
Contact:
Werner Kühlbrandt, Max Planck Institute of Biophysics, Frankfurt/Main, Germany, werner.kuehlbrandt@biophys.mpg.de
Publication:
Thorsten Blum, Alexander Hahn, Thomas Meier, Karen Davies and Werner Kühlbrandt (2019) Dimers of mitochondrial ATP synthase induce membrane curvature and self-assemble into rows. Proceedings of the National Academy of Sciences of the USA, published online 13 February 2019. http://dx.doi.org/10.1073/pnas.1816556116
&nb

