News Archive
Antigenic peptide recognition on the human ABC transporter TAP resolved by DNP-enhanced solid-state NMR spectroscopy
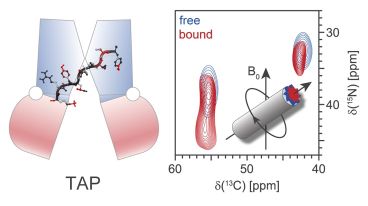
September 2016. The human transporter associated with antigen processing (TAP) selects peptides for export into the endoplasmic reticulum and subsequent loading onto major histocompatibility complex class I molecules to trigger adaptive immune responses against virally or malignantly transformed cells. Up to date, no atomic-resolution information on the peptide-TAP interactions has been obtained, hampering the mechanistic understanding of the early steps of substrate translocation catalyzed by TAP.
In a tour-de-force and large collaborative effort, a Frankfurt team including the Glaubitz, Hummer and Tampé labs developed a new preparation method to concentrate an unstable membrane protein and combined this effort with dynamic nuclear polarization (DNP) enhanced magic angle spinning (MAS) solid-state NMR to determine the atomic-resolution backbone conformation of an antigenic peptide bound to human TAP.
The findings reveal a structural and chemical basis of substrate selection rules, which define the crucial function of this ABC transporter in human immunity and health. This work is the first NMR study of a eukaryotic transporter protein and presents the power of solid-state NMR in this growing field. More ...
Contacts:
Robert Tampé
Institute of Biochemistry, Biocenter, Goethe University Frankfurt, Germany, tampe@em.uni-frankfurt.de
Clemens Glaubitz
Institute of Biophysical Chemistry, Biocenter, Goethe University Frankfurt, Germany, glaubitz@em.uni-frankfurt.de
Publication:
Lehnert E, Mao J, Mehdipour AR, Hummer G, Abele R, Glaubitz C, Tampé R (2016) Antigenic peptide recognition on the human ABC transporter TAP resolved by DNP-enhanced solid-state NMR spectroscopy. J Am Chem Soc, Epub ahead of print 23 September 2016 http://dx.doi.org/10.1021/jacs.6b07426