News Archive
Development of a synthetic TAP inhibitor that can be cleaved by light
December 2018. The immune system is one of the most complex systems in the human body – with many different types of immune cells as well as transport and messenger molecules - but pathogens and cancer cells manage to outwit it again and again. Viruses, for example, have developed ingenious weapons to block the body’s immune system. A team of researchers from the Institute of Biochemistry at Goethe University Frankfurt now turned one of these weapons into a tool to study specific details of the immune response that we do not understand yet.
It is known that cells provide the T cells of the adaptive immune system with information about their condition by presenting selected components of their interior (antigens) on their surface. If these components include fragments of viruses, bacteria or altered cell components, the affected cell is eliminated by the T cells. Although many steps in antigen processing have been elucidated, it is less clear how the events are linked and how the flux of peptides is coordinated in space and time. New tools are therefore needed which allow control of specific check-points in the antigen processing pathway with high accuracy in a time and space-resolved manner.
Researchers from the Institute of Biochemistry at Goethe University Frankfurt used the virus protein ICP47 to develop such a tool. ICP47 is produced by the herpes simplex virus and represents one of the most potent immune suppressors. In the cell it binds to the transporter associated with antigen processing - called `TAP´ - which is the central assembly unit of a large macromolecular complex called the `peptide-loading complex´ (`PLC´).
PLC is a transient, multisubunit membrane complex in the endoplasmic reticulum that is essential for establishing a hierarchical immune response. This complex coordinates peptide translocation into the endoplasmic reticulum with loading and editing of the major histocompatibility complex (MHC-I). After final proofreading, stable peptide–MHC-I complexes are released to the cell surface to evoke a T-cell response against infected or malignant cells.
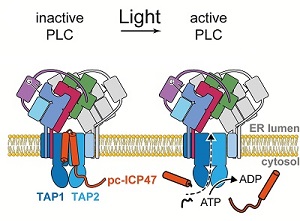
The Frankfurt scientists synthesized a modified version of the viral protein ICP47 to make the inhibitor cleavably by light. This photo-cleavable version of ICP47 - named `pc-ICP47´ by the scientists - was designed by substituting one of the natural amino acid of the virus protein with the photo-cleavable amino acid Anp. This inhibitor pc-ICP47 blocks TAP-mediated antigen translocation but when exposed to light of a wavelength of 365 nm, the inhibitor is split into two fragments and does not block TAP anymore.
The scientists carefully monitored the recovery of TAP activity at single-cell resolution both in human immune cell lines and blood-derived primary cells. The new tool provides fast photo-activation along with a clean fragmentation, both are essential for a precise control when conducting experiments. The development of a photo-cleavable TAP inhibitor thus expands the repertoire of chemical intervention tools for the study of immunological processes. More ...
Contacts:
Robert Tampé (tampe@em.uni-frankfurt.de) and Ralph Wieneke (wieneke@em.uni-frankfurt.de), Institute of Biochemistry, Riedberg Campus, Goethe University Frankfurt, Frankfurt/Main, Germany
Publication:
Markus Braner, Nicole Koller, Julia Knauer, Valentina Herbring, Susanne Hank, Ralph Wieneke* and Robert Tampé* (2019) Optical control of the antigen translocation by syntethic photo-conditional viral inhibitors. Published online in Chemical Science on 17 December 2018. http://dx.doi.org/10.1039/C8SC04863K
Cluster of Excellence Macromolecular Complexes, Frankfurt am Main, Germany

