News Archive
X-ray structural analysis provides new insights into the mode of action of respiratory complex I
2 July 2010
Proton-pumping respiratory complex I is among the largest and most complicated membrane protein complexes. Its function is critical for efficient energy supply in aerobic cells and malfunctions are implicated in many neurodegenerative disorders. Volker Zickermann in the group of Ulrich Brandt together with former CEF member Carola Hunte now published the X-ray crystallographic analysis of mitochondrial complex I in the journal Science. The positions of all iron-sulfur clusters relative to the membrane arm were determined in the complete enzyme complex. The ubiquinone reduction site resides close to 30 Å above the membrane domain. The arrangement of functional modules suggests conformational coupling of redox chemistry with proton pumping and essentially excludes direct mechanisms. The team suggests that a ~60 Å long helical transmission- element is critical for transducing conformational energy to proton- pumping elements in the distal module of the membrane arm.
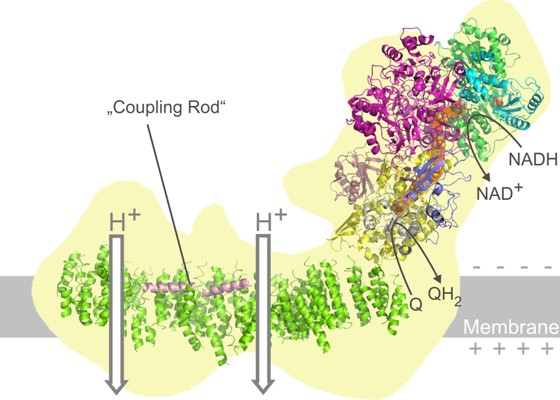
Figure: The structural model of mitochondrial complex I has important implications for its function: In the upper right part bound hydrogen is transferred from NADH to coenzyme Q. During this process electrons are transferred through a series of so called iron-sulfur clusters (orange). The hydrogen transfer drives two proton pumps in the membrane domain of the giant multiprotein enzyme complex. The pumping modules are connected via a molecular “coupling rod”. The transfer of charges results in an electrical potential across the membrane that is then used by complex V of the respiratory chain to make ATP (not shown).
Contact for further information: Ulrich Brandt, Molecular Bioenergetics, Cluster of Excellence Makromolecular Complexes, Niederrad Campus , Frankfurt am Main, Germany, Tel. +49 (0)69 6301-6925; brandt@zbc.kgu.de.
Full reference: Carola Hunte, Volker Zickermann and Ulrich Brandt. 2010. Functional modules and structural basis of conformational coupling in mitochondrial complex I. Science. Published online 1 July 2010, DOI: 10.1126/science.1191046 . More ...

