News Archive
The control of signaling in immunity and inflammation
April 2014. The groups of Ivan Dikic and Masato Akutsu have moved closer in understanding how a novel form of protein modification, the linear ubiquitination, controls central pathways of immunity and inflammation. In a collaborative effort, they showed that the two enzymes responsible for assembling and disassembling linear ubiquitin chains are contained in one complex. 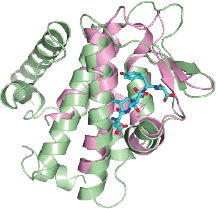
By structural analysis, they managed to decipher the molecular details of the interaction between these two key enzymes and were able to show how the opposing activities of the complex are controlled. Their results are published in the current edition of Molecular Cell online. More...
Linear ubiquitin chains are implicated in the regulation of the NF-κB pathway, immunity, and inflammation. They are synthesized by the LUBAC complex containing the catalytic subunit HOIL-1-interacting protein (HOIP) and are disassembled by the linear ubiquitin-specific deubiquitinase OTULIN. The scientists demonstrate that HOIP and OTULIN interact and act as a bimolecular editing pair for linear ubiquitin signals in vivo. The HOIP PUB domain binds to the PUB interacting motif (PIM) of OTULIN and the chaperone VCP/p97. Structural studies revealed the basis of high-affinity interaction with the OTULIN PIM. The conserved Tyr56 of OTULIN makes critical contacts with the HOIP PUB domain, and its phosphorylation negatively regulates this interaction. Functionally, HOIP binding to OTULIN is required for the recruitment of OTULIN to the TNF receptor complex and to counteract HOIP-dependent activation of the NF-κB pathway.
Full reference:
Schäffer V, Akutsu M, Olma MH, Gomes LC, Kawasaki M, Dikic I (2014) Binding of OTULIN to the PUB domain of HOIP controls NF-κB signaling. Mol Cell: Published Online 10 April 2014, doi: 0.1016/j.molcel.2014.03.016. Link to full paper
Contact:
Ivan Dikic
Institute of Biochemistry II
Frankfurt (Main), Germany
Tel.: +49 (0)69 6301-5964
ivan.dikic@biochem2.de

