News Archive
Novel regulatory mechanism for ligand-induced action at a distance
May 2017. Ion channel gating is essential for cellular homeostasis and is tightly controlled. In some eukaryotic and most bacterial ligand-gated K+ channels, RCK domains regulate ion fluxes. Until now, a single regulatory mechanism has been proposed for all RCK-regulated channels, involving signal transduction from the RCK domain to the gating area.
Scientists from Goethe University Frankfurt, Max Planck Institute of Biophysics and Osnabrück University now report a new mechanism. In the journal eLife they present an inactive ADP-bound structure of the potassium transporter KtrAB from Vibrio alginolyticus, determined by cryo-electron microscopy. Combined with EPR spectroscopy and molecular dynamics simulations, this structure uncovers a novel regulatory mechanism for ligand-induced action at a distance. As KtrAB and its homolog TrkAH have been implicated as bacterial pathogenicity factors, the discovery of this functionally relevant inactive conformation may advance structure-guided drug development.
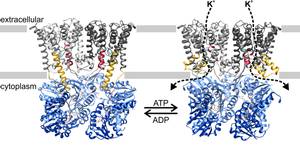
Exchange of activating ATP to inactivating ADP triggers short helical segments in the K+-translocating KtrB dimer to organize into two long helices that penetrate deeply into the regulatory RCK domains, thus connecting nucleotide binding sites and ion gates:
Figure: KtrAB activation. Structural models of the ADP- and ATP-bound conformations. With ADP bound, the extended helices D1M2b of KtrB dimers interact with residues in KtrAs close to the ADP binding sites, and with the gating regions of KtrBs. The gates are locked in the closed conformation. In the presence of ATP, the D1M2 helices break, forming helix hairpins at the membrane surface, and helices D4M2b move away from the pore. The disruption of interactions in the gating regions allows the intramembrane loops to move. In general, K+ flux is enabled. KtrAs, blue; KtrBs, grey with helices D1M2b, yellow; helices D4M2b, magenta; and intramembrane loop, green. More ...
Contact:
Inga Hänelt, Institute of Biochemistry, Goethe University Frankfurt, haenelt@biochem.uni-frankfurt.de
Janet Vonck, Max Planck Institute of Biophysics, Frankfurt/Main, janet.vonck@biophys.mpg.de
Publication:
Diskowski M, Mehdipour AR, Wunnicke D, Mills DJ, Mikusevic V, Bärland N, Hoffmann J, Morgner N, Steinhoff H-J, Hummer G, Vonck J*, Haenelt I* (2017) Helical jackknives control the gates of the double-pore K+ uptake system KtrAB. eLife 6: e24303. http://dx.doi.org/10.7554/eLife.24303
Cluster of Excellence Macromolecular Complexes, Frankfurt am Main, Germany