News Archive
The way Carnitin enters cells
Transport of solutes across biological membranes is performed by specialized secondary transport proteins in the lipid bilayer, and is essential for life. A team of scientists at the Max Planck Institute of Biophysics now reports the structures of the sodium-independent carnitine/butyrobetaine antiporter CaiT from Proteus mirabilis (PmCaiT) at 2.3-angstrom and from Escherichia coli (EcCaiT) at 3.5-angstrom resolution. CaiT belongs to the family of betaine/carnitine/choline transporters (BCCT), which are mostly Na+ or H+ dependent, whereas EcCaiT is Na+ and H+ independent. The three-dimensional architecture of CaiT resembles that of the Na+-dependent transporters LeuT and BetP, but in CaiT a methionine sulphur takes the place of the Na+ ion to coordinate the substrate in the central transport site, accounting for Na+-independent transport. Both CaiT structures show the fully open, inward-facing conformation, and thus complete the set of functional states that describe the alternating access mechanism. EcCaiT contains two bound butyrobetaine substrate molecules, one in the central transport site, the other in an extracellular binding pocket. In the structure of PmCaiT, a tryptophan side chain occupies the transport site, and access to the extracellular site is blocked. Binding of both substrates to CaiT reconstituted into proteoliposomes is cooperative, with Hill coefficients up to 1.7, indicating that the extracellular site is regulatory. The team proposes a mechanism whereby the occupied regulatory site increases the binding affinity of the transport site and initiates substrate translocation.
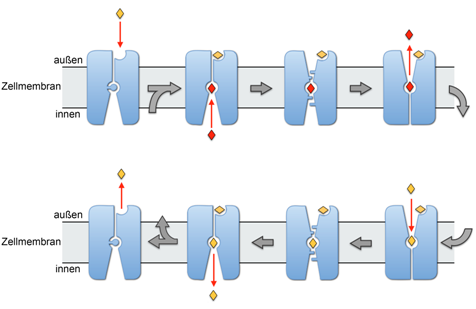
The empty transporter, represented by the PmCaiT structure, is activated by l-carnitine (yellow diamond) binding to the regulatory site. This triggers the re-orientation of Trp 323 (blue disk), and γ-butyrobetaine (red diamond) binds to the central transport site from the cytoplasm. The state in which both binding sites are occupied is represented by the EcCaiT structure. In the occluded state, represented by the BetP structure4, the substrate in the transport site is inaccessible. In the outside-open state, represented by the LeuT structure6, γ-butyrobetaine in the central transport site is replaced by l-carnitine. CaiT then changes conformation through the occluded state back to the inside-open state, releasing l-carnitine to the cytoplasm, where it is metabolized. The next γ-butyrobetaine product binds, and the transport cycle repeats. When the outside l-carnitine concentration drops below a critical level, the substrate diffuses out of the regulatory site, and the transporter switches off. Publikation: Sabrina Schulze et. al. Structural Basis of Na+-independent and cooperative substrate/product antiport in CaiT, Nature, Band 467, S. 233, doi:10.1038. more ...
.
Contacts for further information:
Sabrina Schulze and Prof. Werner Kühlbrandt, Max Planck Institute of Biophysics, Cluster of Excellence Macromolecular Complexes Frankfurt, Riedberg Campus
Tel.: +49 69 6303-3054, sabrina.schulze@biophys.mpg.de
Tel.: +49 69 6303-3000, werner.kuelbrandt@biophys.mpg.de