News Archive
New insights into the remodelling of mitochondria
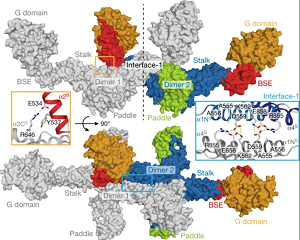 July 2019. Structural and functional studies reported this month in the journal Nature reveal the molecular basis of remodelling of the inner mitochondrial membrane by the protein complex Mgm1.
July 2019. Structural and functional studies reported this month in the journal Nature reveal the molecular basis of remodelling of the inner mitochondrial membrane by the protein complex Mgm1.
Mitochondria are the powerhouses of our cells and play a fundamental role in human health. Balanced fusion and fission are essential for the proper function and physiology of these important organelles.
Mitochondria possess two membranes. In humans and animals remodelling of the inner mitochondrial membrane is mediated by a protein complex called OPA1. The related protein complex Mgm1 takes on this role in yeasts and other fungi. Both Mgm1 and OPA1 exist in mitochondria in two forms: a membrane-integrated long form and a short form that is soluble in the intermembrane space.
Mutations in the OPA1 gene in humans are a common cause of autosomal dominant optic atrophy—a genetic disorder that affects the optic nerve. Mammalian cells that lack OPA1 and yeast strains that express temperature-sensitive versions of Mgm1 have fragmented mitochondria. This suggests that Mgm1 and OPA1 have an important role in inner-membrane fusion. Only the mitochondrial outer membrane — not the inner membrane — fuses in the absence of functional Mgm1.
Mgm1 and OPA1 have also been shown to maintain proper cristae architecture. For example, OPA1 prevents the release of pro-apoptotic factors by tightening crista junctions. And the short form of OPA1 localizes to mitochondrial constriction sites, where it presumably promotes mitochondrial fission. How Mgm1 and OPA1 perform their diverse functions in membrane fusion, scission and cristae organization is at present unknown.
A team of scientists led by Werner Kühlbrandt from the Max Planck Institute of Biophysics in Frankfurt and Oliver Daumke from the Max-Delbrück-Centrum for Molecular Medicine in Berlin has solved the crystal and electron cryo-tomography structures of Mgm1 from the fungus C. thermophilum. The structural and functional studies they present in the journal Nature this month reveal the molecular basis of the assembly of Mgm1 into filaments, and provide models of how the rearrangements of these filaments induce remodelling of the inner mitochondrial membrane.
Mgm1 consists of a GTPase (G) domain, a bundle signalling element domain, a stalk, and a paddle domain that contains a membrane-binding site. Biochemical and cell-based experiments demonstrated that the Mgm1 stalk mediates the assembly of bent tetramers into helical filaments. Their electron cryo-tomography studies of Mgm1-decorated lipid tubes and fluorescence microscopy experiments on reconstituted membrane tubes indicate how the tetramers assemble on positively or negatively curved membranes. More...
Contact:
Werner Kühlbrandt, Max Planck Institute of Biophysics, Frankfurt am Main, werner.kuehlbrandt@biophys.mpg.de
Publication:
Katja Faelber, Lea Dietrich, Jeffrey K. Noel, Florian Wollweber, Anna-Katharina Pfitzner, Alexander Mühleip, Ricardo Sánchez, Misha Kudryashev, Nicolas Chiaruttini, Hauke Lilie, Jeanette Schlegel, Eva Rosenbaum, Manuel Hessenberger, Claudia Matthaeus, Séverine Kunz, Alexander von der Malsburg, Frank Noé, Aurélien Roux, Martin van der Laan, Werner Kühlbrandt* & Oliver Daumke* (2019)
Structure and assembly of the mitochondrial membrane remodelling GTPase Mgm1.
published online 10 July 2019 in Nature
http://dx.doi.org/10.1038/s41586-019-1372-3

