News Archive
Unprecedented mechanism in bacterial efflux pump revealed
16 October 2014. Membrane transporters of the RND superfamily confer multidrug resistance to pathogenic bacteria, and are essential for cholesterol metabolism and embryonic development in humans. A team of scientists led by Martin Pos and José Faraldo-Gómez used high-resolution X-ray crystallography and computational methods to delineate the mechanism of the homotrimeric RND-type proton/drug antiporter AcrB, the active component of the major efflux system AcrAB-TolC in Escherichia coli, and one most complex and intriguing membrane transporters known to date. Analysis of wildtype AcrB and four functionally-inactive variants revealed an unprecedented mechanism that involves two remote alternating-access conformational cycles within each protomer, namely one for protons in the transmembrane region and another for drugs in the periplasmic domain, 50 Å apart. Each of these cycles entails two distinct types of collective motions of two structural repeats, coupled by flanking α-helices that project from the membrane.
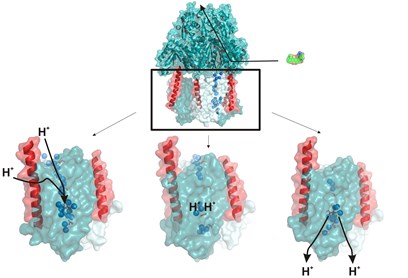
Top: The AcrB pump used by the E. coli bacterium to remove antibiotics (green) from its cell. This process is driven by a gradient of protons (H+). The energy released is transferred from the transmembrane domain (outlined in black) through two helices (red) to the antibiotic-transporting domain.
Below: The three different conformations of the transmembrane domain.
Contact:
Klaas Martinus Pos, Institute of Biochemistry and Cluster of Excellence Macromolecular Complexes, Riedberg Campus, Goethe University Frankfurt
Tel.: +49 (0)69 798- 29251, pos@em.uni-frankfurt.de
www.biochem.uni-frankfurt.de/index.php?id=7 und http://www.sfb807.de/klaas-martinus-pos.html
Publication
Eicher, T., Seeger, M. A.., Anselmi, C., Zhou, W., Brandstätter, L., Verrey, F., Diederichs, K., Faraldo-Gómez, J. D.*, Pos, K. M.* (2014) Coupling of remote alternating-access transport mechanisms for protons and substrates in the multidrug efflux pump AcrB. eLife 3:e03145.
elifesciences.org/content/3/e03145

