News Archive
New insights into ribosome recycling with the enzyme ABCE1
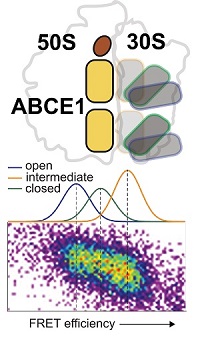 July 2019. Ribosomes translate messenger RNA (mRNA) into proteins and are present in large numbers in all living cells. The protein ABCE1, a twin-ATPase, recycles terminated or stalled ribosomes and therefore plays an important role in the quality control of mRNA translation. By using an integrated biophysical approach, an international team of scientsts from Groningen, Leuven, Munich and Frankfurt was able to directly observe for the first time the conformational dynamics and ribosome association of ABCE1 at the single-molecule level.
July 2019. Ribosomes translate messenger RNA (mRNA) into proteins and are present in large numbers in all living cells. The protein ABCE1, a twin-ATPase, recycles terminated or stalled ribosomes and therefore plays an important role in the quality control of mRNA translation. By using an integrated biophysical approach, an international team of scientsts from Groningen, Leuven, Munich and Frankfurt was able to directly observe for the first time the conformational dynamics and ribosome association of ABCE1 at the single-molecule level.
Their results indicate that the current static two-state model of ABC proteins has to be expanded, because the two ATP sites of ABCE1 are in dynamic equilibrium across three distinct conformational states: open, intermediate and closed. The interaction of ABCE1 with ribosomes influences the conformational dynamics of both ATP sites asymmetrically and creates a complex netowork of conformational states.
ABCE1 belongs to the large and ubiquitous family of ABC proteins. The new findings represent a paradigm shift to redefine understanding of the mechanochemical coupling in ABC proteins. More...
Contact:
Robert Tampé, Institute of Biochemistry, Riedberg Campus, Goethe University, Frankfurt/Main, Germany, tampe@em.uni-frankfurt.de
Publication:
Giorgos Gouridis, Bianca Hetzert, Kristin Kiosze-Becker, Marijn de Boer, Holger Heinemann, Elina Nürenberg-Goloub, Thorben Cordes* & Robert Tampé* (2019) ABCE1 controls ribosome recycling by an asymmetric dynamic conformational equilibrium. Cell Reports 28, published online 16 July 2019, doi 10.1016/j.cellrep.2019.06.052 (Link)

