News Archive
New proteins for new memories
February 2018. Researchers at the Max Planck Institute for Brain Research have found a way to measure newly synthesized proteins in artificially grown nerve cells from the rat hippocampus after regulating their connectivity for a certain amount of time. The researchers observed for the first time that conditions, such as time and up- or down regulation, determine which new proteins were synthesized.
Each time the brain stores information, it changes the strength at the synapses, the connections between nerve cells. When two neurons at a synapse are active at the same time, they will become stronger connected, enabling the first neuron to activate the second even more easily. If this process continues unchecked, the synaptic strength will reach a threshold value, which will make it harder for the brain to form new memories. To prevent this from happening, the brain responds to prolonged changes in the activity of neurons by adjusting the strength of synapses in the opposite direction. If neurons are too active for an extended period of time, the brain reduces the strength of synapses. The brain can also do the opposite when neurons show too little activity. This regulatory process is known as homeostatic scaling, and the brain achieves this by adjusting the number and/or type of proteins present at synapses.
In order to track changes in proteins after manipulating the synaptic strength, researchers grew cells in a petri dish in the presence of modified building blocks. Christoph Schanzenbächer: “All new proteins will contain these amino acids, making them easy to spot.” The Max Planck researchers applied this technique to neurons obtained from the rat hippocampus, a region of the brain involved in learning and memory. Bathing the neurons for two hours in chemicals that either enhanced or reduced their activity triggered changes in more than 150 proteins.
They now compared these results to those of a previous experiment in which neuronal activity had been manipulated for 24 hours. Surprising, each set of conditions produced a distinct, characteristic profile of protein expression. This allows researchers to “read-out” from the system whether the network was enhanced or decreased and for how long. Erin Schuman: “This is the first study of its kind to monitor the dynamics of brain proteins, or the ‘proteome’ during plasticity. These findings may provide insights into disease states in which there is too much or too little brain activity.”
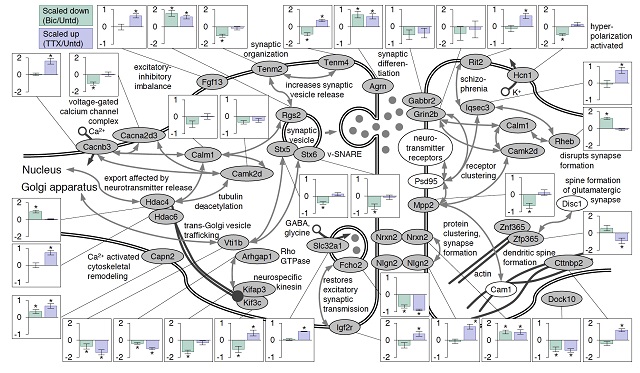
Figure: Proteins at neuronal synapses exhibit changes in expression levels following prolonged increases or decreases in network activity
Contact:
Erin M. Schuman, Max Planck Institute for Brain research, Max-von-Laue-Str. 4, 60438 Frankfurt am Main, Germany, e-mail: erin.schuman@brain.mpg.de, tel.: +49 69 850 033 1001 (Nicole Thomson)
Publication:
Schanzenbächer CT, Langer JD, Schuman EM (2018) Time- and polarity-dependent proteomic changes associated with homeostatic scaling at central synapses. eLife 7: http://dx.doi.org/10.7554/eLife.33322

