News Archive
New ubiquitin chemistry regulates life processes
December 2016. In the latest issue of the journal Cell, a team around IBC2 director and CEF member Ivan Dikic reveals molecular details of a novel ubiquitination mechanism that may affect numerous life processes. Earlier this year, U.S. colleagues reported that the enzyme SdeA from the pathogenic bacterium Legionella is capable of catalysing ubiquitination single-handedly. Now, the Frankfurt scientists together with collaborators from the MPI for Biology of Ageing (Cologne) have elucidated the chemistry behind this process and discovered a hitherto unknown type of linkage between ubiquitin and target proteins. Unlike the conventional ubiquitination reaction, the novel reaction is NAD-dependent, involving an ADP-ribose intermediate and resulting in the attachment of ubiquitin to substrate serine residues via a phosphodiester bond.
While these findings alone are breaking new ground, the discovery went even further: The team showed that the Legionella enzyme does not only transfer ubiquitin onto target proteins, but also leaves behind a complete pool of chemically modified, phosphoribosylated ubiquitin. Phosphoribosylated ubiquitin almost completely inhibits the conventional ubiquitination system and thereby affects essential cellular processes, e.g. proteasomal protein degradation, mitophagy and pro-inflammatory signalling. This explains the pathogenic effects of Legionella infection in immunocompromised patients, who often suffer from extensive lung tissue damage despite antibiotic treatment. The insights generated by the Dikic team may now open the road to the development of new antibacterial agents, which could complement conventional antibiotics by limiting the cellular damage induced by bacterial enzymes.
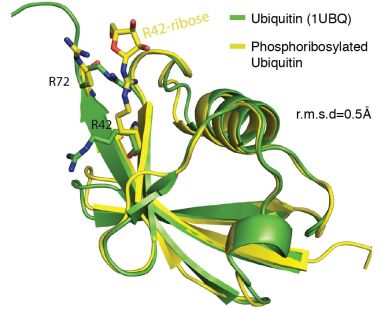
Figure: Superposed crystal structures of (i) normal ubiquitin (green) and (b) ubiquitin modified by the Legionella enzyme (yellow). Modified ubiquitin contains an additional phosphoribosyl group at amino acid position 42 (Cell 2016, 167(6)).
Publication:
Bhogaraju S, Kalayil S, Liu Y, Bonn F, Colby T, Matic I, Dikic I. 2016. Phosphoribosylation of ubiquitin promotes serine ubiquitination and impairs conventional ubiquitination. Cell 167(6). DOI 10.1016/j.cell.2016.11.019. Link
Contact:
Kerstin Koch, Institute of Biochemistry II, Goethe University School of Medicine, Frankfurt/Main, Germany, Tel.: +49 69 6301 84250, koch@biochem2.de
Link to Cell paper
Link to German press release
twitter
facebook

