News Archive
Functional cooperativity of dimers of an important solute carrier family
May 2019. By integrating PELDOR measurements and in vitro transport studies with structural modeling and refinement using molecular dynamics simulation, Frankfurt scientists determined the architecture of an important type of membrane-embedded cellular solute carrier and characterized its functional relevance.
The solute carrier family 26 (SLC26) facilitates the transport of a broad variety of organic and inorganic anions in cells. Members of this family of protein complexes are found in all kingdoms of life and operate predominantly as secondary transporters (symporters and exchangers). The relevance of the SLC26 family in maintaining anion equilibria is underlined by the causative role of mammalian SLC26 proteins in diseases such as congenital chloride diarrhea and cytotoxic brain edema.
The SLC26 family shares a 7 + 7 transmembrane segments inverted repeat architecture (7TMIR) with the SLC4 and SLC23 families, but holds a regulatory STAS domain in addition. While the only experimental SLC26 structure is monomeric, SLC26 proteins form structural and functional dimers in the lipid membrane. Scientists of Goethe University Frankfurt and the Max Planck Institute of Biophysics solved the structure of an SLC26 dimer embedded in a lipid membrane and characterized its functional relevance by combining pulsed electron–electron double resonance (PELDOR/DEER) distance measurements and biochemical studies with molecular dynamics simulations and spin-label ensemble refinement.
Their structural model reveals a unique interface different from the SLC4 and SLC23 families. The functionally relevant STAS domain is no prerequisite for dimerization. Characterization of heterodimers indicates that protomers in the dimer functionally interact. These combined structural and functional data define the framework for a mechanistic understanding of functional cooperativity in SLC26 dimers.
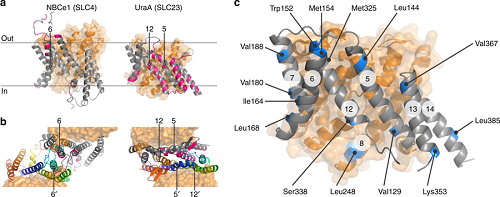
Figure legend: Dimer interfaces in 7TMIR proteins. a Side view of the membrane domains of the transporters NBCe1 and UraA. Core and gate domain are colored orange and gray, respectively, with residues within 4 Å of the opposing protomer in pink. b Top views of the dimeric arrangements of NBCe1 and UraA. For each dimer, the gate domain of one of the protomers follows a rainbow coloring scheme (blue-to-red for N-to-C direction). Transmembrane segments central in the respective dimers are numbered. c Side view of the membrane domain of SLC26Dg. Residues mutated to cysteine for site-directed spin labeling are colored blue. The circled numbers indicate the respective transmembrane segments (Nature Communications 2019, Vol. 10, Article no. 2032)
Contacts:
Eric R. Geertsma, Institute of Biochemistry, Goethe University Frankfurt, geertsma@em.uni-frankfurt.de, and Gerhard Hummer, Dept of Theoretical Biophysics, Max Planck Institute of Biophysics, gerhard.hummer@biophys.mpg.de, Riedberg Campus, Frankfurt/Main, Germany
Publication:
Yung-Ning Chang, Eva A. Jaumann, Katrin Reichel, Julia Hartmann, Dominik Oliver, Gerhard Hummer*, Benesh Joseph* & Eric R. Geertsma* (2019) Structural basis for functional interactions in dimers of SLC26 transporters. Nature Communications 10: 2032. http://dx.doi.org/10.1038/s41467-019-10001-w