News Archive
Presenilin and the development of Alzheimer
30 April 2010 - FRANKFURT. The increase in life expectancy in the industrialized states has resulted in an increase in the number of patients suffering from dementia. 60% of these dementia cases are due to Alzheimer’s disease which is caused by the death of certain neurons in the brain. Characteristic for Alzheimer’s disease is deposition of plaques consisting of amyloid beta peptide. According to the current model it is not these plaques but rather oligomeric and soluble precursors that are responsible for neuronal death. If the formation of the beta amyloid protein could be suppressed, the production of these oligomers would be suppressed as well. Central to the process of amyloid beta peptide formation is the enzymatic activity of presenilin. So far the structure of this protease was not available. As typical for membrane proteins, characterization of its structure met great difficulties with the well established classical methods of x-ray crystallography or NMR spectroscopy. A research team headed by Volker Dötsch was now successful in determining the structure of the C-terminal domain of presenilin, as they report in the Proceedings of the US National Academy of Sciences.
The topology of this C-terminal domain that harbors one of the two catalytic aspartate residues was so far highly controversial and predictions were based on computational modeling and indirect biochemical experiments. To investigate the structure of the C-terminal domain the research group of Volker Dötsch used a method that has been developed at the Institute of Biophysical Chemistry for several years. The group of Frank Bernhard had shown that membrane proteins can be obtained in the large amounts required for detailed structural studies by isolating the protein synthesis machinery from E. coli and reconstituting protein synthesis in vitro in a test tube. This method of protein production enabled the development of novel NMR techniques that made the investigation of membrane proteins in isolated micelles possible.
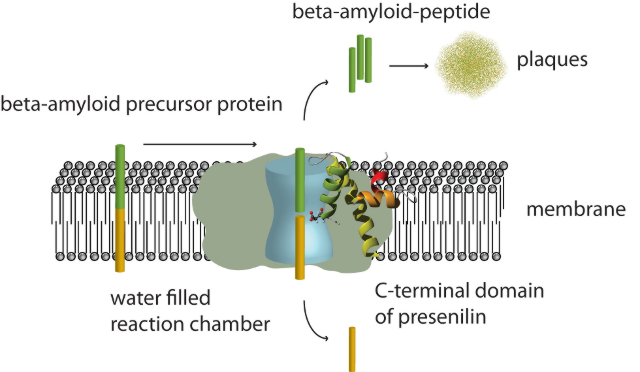
The figure shows the process of plaque formation in the brains of Alzheimer patients. The membrane bound amyloid precursor protein gets cleaved into two parts by the membrane embedded enzymatic γ-secretase complex. The extracellular part (shown in green) first forms soluble oligomers which further aggregate to create the characteristic plaques. So far only the overall shape of the entire γ-secretase complex (depicted as gray surface) and the existence of a water filled cavity in its interior (light blue) was known. The newly determined structure of the C-terminal catalytic domain of presenilin 1 now demonstrates that the active site aspartate is located in the center of the membrane, however, part of the water filled cavity in the interior of the proteolytic complex.
The structure of the C-terminal domain now solved is the first high resolution structure of any of the components of the γ-secretase, the multi-component membrane protein complex that is responsible for producing the amyloid beta peptide by proteolytically cutting the amyloid beta precursor protein and of which presenilin is the most important component. The structure shows that the C-terminal domain consists of three helical regions that traverse the membrane. Of these, however, only one is a typical transmembrane helix – a fact that explains the difficulty in predicting the topology of this domain by pure computational approaches. The catalytic aspartate is located at the N-terminus of one of the helices which, however, is too short to fully traverse the membrane, thus placing the catalytic residue in the middle of the membrane. However, by additional experiments the research group of Volker Dötsch could show that the aspartate is surrounded by a hydrophilic environment. This finding is consistent with the results from single particle cryo-electron microscopy of the entire γ-secretase complex which has shown that in the center of this enzyme complex a large water filled cavity exists in which proteolysis is believed to take place (Lazarov et al., PNAS 2006; Osenkowski et al., JMB 2009). The final transmembrane helix of the C-terminal domain shows a highly kinked and unusual structure due to the presence of a proline in the middle of the helical region. This unusual helical structure has been implicated in being involved in substrate binding and a very similar structural element has been identified in the structure of the rhomboid protease, a bacterial membrane bound enzyme.
In addition to these three membrane embedded helical regions the structure revealed the presence of another helix in a long so far believed to be completely unstructured loop that lies at the interface between the cytoplasm and the membrane. Interestingly, several mutations implicated in early onset familial Alzheimer disease are located in this newly discovered helix.
Publication: Sobhanifar S, Schneider B, Löhr F, Gottstein D, Ikeya T, Mlynarczyk K, Pulawski W, Ghoshdastider U, Kolinski M, Filipek S, Güntert P, Bernhard F, Dötsch V (2010) P Natl Acad Sci USA. 2010 [Epub ahead of print]
http://www.ncbi.nlm.nih.gov/pubmed/20445084
Information: Prof. Volker Dötsch, Institute for Biophysical Chemistry and Frankfurt Institute of Molecular Life Sciences (FMLS), Riedberg Campus, Goethe University Frankfurt, Tel.: +49 (0)69 798-29631, vdoetsch@em.uni-frankfurt.de
Links:
Paper
Volker Dötsch's Homepage
Print News Alzforum.org

