News Archive
Characteristics of human lysozyme and its disease-related mutants in their unfolded states
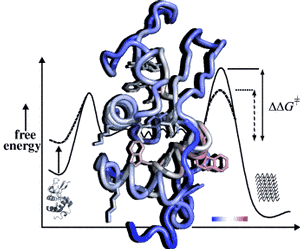
18 May 2011.Point mutations of the very stable human protein lysozyme can trigger kg deposition of amyloid plagues invarious organ. In many protein-folding diseases, single-point mutations, often inherited, are linked to specific disease forms but the structural code for the onset of the disease is still unclear. In a recent publication, the Schwalbe group could delineate how a point mutation of the protein modulate structure and dynamics of unfolded and partially folded states of human lysozyme using liquid state NMR spectroscopy at atomic resolution. The degree of residual structure correlates with the ability of the protein to form amyloid fibrils. The free-energy landscape connecting different members of the ensemble of premolten protein states is affected by single-point mutations.
Link to full publication Angewandte Chemie
Full reference
Sziegat F, Wirmer-Bartoschek J, Schwalbe H (2011) Characteristics of human lysozyme and its disease-related mutants in their unfolded states. Angew Chem Int Edit:in press

