News Archive
Mechanism for precise targeting of the immune response uncovered
October 2017. The immune system monitors the health status of the cells in our bodies by examining a kind of molecular passport. Sometimes cells present the wrong passport, which can lead to autoimmune diseases, chronic inflammation, or cancer. In the new issue of the journal Science (first release), scientists of the Goethe University Frankfurt have now elucidated the mechanism of how the correct molecular passport is selected.
Most cells provide the T cells of the adaptive immune system with information about their condition by presenting selected components of their interior (antigens) on their surface. If these components include fragments of viruses or altered cell components, the affected cell is eliminated by the T cells. The selection of the antigens is crucial in this process. Presenting the wrong antigens leads to either healthy cells being attacked by the immune system - causing autoimmune diseases or chronic inflammation - or to diseased cells not being recognized, allowing cancer cells or virus-infected cells to escape immune surveillance.
Christoph Thomas and Robert Tampé from the Institute of Biochemistry at Goethe University have now unraveled on a molecular level how antigens are selected in the cell for presentation on the cell surface. The protein structure they present shows for the first time the kind of quality control antigens undergo to enable a precise and effective immune response.
“Our work solves a 30-year-old problem of cellular immunity, in particular how antigens associated with tumors or pathogens are selected through processes of editing and quality control in order to generate a specific immune response” explains Robert Tampé the significance of the publication.
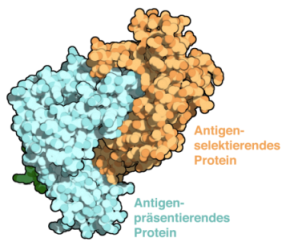 Adaptive immunity is shaped by a selection of peptides presented on major histocompatibility complex class I (MHC I) molecules. The chaperones Tapasin (Tsn) and TAP- binding protein-related (TAPBPR) facilitate MHC I peptide loading and high-affinity epitope selection. Despite the pivotal role of Tsn and TAPBPR in controlling the hierarchical immune response, their catalytic mechanism remains unknown. The Frankfurt scientists solved the X-ray structure of the TAPBPR–MHC I complex, which delineates the central step of catalysis. TAPBPR functions as peptide selector by remodeling the MHC I α2-1-helix region, stabilizing the empty binding groove, and inserting a loop into the groove that interferes with peptide binding. The complex explains how mutations in MHC I-specific chaperones cause defects in antigen processing and suggests a unifying mechanism of peptide proofreading. More...
Adaptive immunity is shaped by a selection of peptides presented on major histocompatibility complex class I (MHC I) molecules. The chaperones Tapasin (Tsn) and TAP- binding protein-related (TAPBPR) facilitate MHC I peptide loading and high-affinity epitope selection. Despite the pivotal role of Tsn and TAPBPR in controlling the hierarchical immune response, their catalytic mechanism remains unknown. The Frankfurt scientists solved the X-ray structure of the TAPBPR–MHC I complex, which delineates the central step of catalysis. TAPBPR functions as peptide selector by remodeling the MHC I α2-1-helix region, stabilizing the empty binding groove, and inserting a loop into the groove that interferes with peptide binding. The complex explains how mutations in MHC I-specific chaperones cause defects in antigen processing and suggests a unifying mechanism of peptide proofreading. More...
Contacts:
Robert Tampé, Tel.: +49 (0)69 798- 29475, tampe@em.uni-frankfurt.de
Christoph Thomas, c.thomas@em.uni-frankfurt.de
Institute of Biochemistry, Campus Riedberg, Goethe University Frankfurt, Germany
Publication:
Christoph Thomas, Robert Tampé: Structure of the TAPBPR–MHC I complex defines the mechanism of peptide loading and editing, Science, published online 12 October 2017 (First Release), dx.doi.org/10.1126/science.aao6001. Link...

