News Archive
Shedding light on ribosome recycling
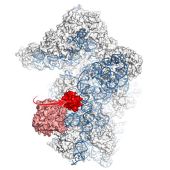 April 2017. Ribosomes carry out all biosynthesis of proteins in living organisms. Cells recycle ribosomes by splitting them into two subunits (called 60S and 40S), a complicated process that is only partly understood. A crucial player is the ABCE1 protein, one of the most conserved proteins in evolution. A study by a team of scientists from Frankfurt and Munich published in the latest issue of the journal Nature Structural & Molecular Biology now sheds new light on the precise role of the ABC1 protein. Using cryo-electron microscopy, the scientists determined the structure of the complex of the 40S subunit with the ABCE1 protein in yeast at high resolution just after the split into the two subunits occurred. Compared to the pre-splitting state, they observed repositioning of ABCE1’s iron-sulfur cluster domain, which rotates 150° into a binding pocket on the 40S subunit. This repositioning explains a newly observed anti-association activity of ABCE1. The movement implies a collision with A-site factors, thus explaining the splitting mechanism. Disruption of key interactions in the post-splitting complex impaired cellular homeostasis. Additionally, the structure of a native post-splitting complex revealed ABCE1 to be part of the ribosomal 43S initiation complex, suggesting that ribosome recycling and translation initiation are linked. More…
April 2017. Ribosomes carry out all biosynthesis of proteins in living organisms. Cells recycle ribosomes by splitting them into two subunits (called 60S and 40S), a complicated process that is only partly understood. A crucial player is the ABCE1 protein, one of the most conserved proteins in evolution. A study by a team of scientists from Frankfurt and Munich published in the latest issue of the journal Nature Structural & Molecular Biology now sheds new light on the precise role of the ABC1 protein. Using cryo-electron microscopy, the scientists determined the structure of the complex of the 40S subunit with the ABCE1 protein in yeast at high resolution just after the split into the two subunits occurred. Compared to the pre-splitting state, they observed repositioning of ABCE1’s iron-sulfur cluster domain, which rotates 150° into a binding pocket on the 40S subunit. This repositioning explains a newly observed anti-association activity of ABCE1. The movement implies a collision with A-site factors, thus explaining the splitting mechanism. Disruption of key interactions in the post-splitting complex impaired cellular homeostasis. Additionally, the structure of a native post-splitting complex revealed ABCE1 to be part of the ribosomal 43S initiation complex, suggesting that ribosome recycling and translation initiation are linked. More…
Contact:
Robert Tampé, Institute of Biochemistry, Goethe University Frankfurt, Frankfurt am Main, Germany, tampe@em.uni-frankfurt.de
Publication:
André Heuer, Milan Gerovac, Christian Schmidt, Simon Trowitzsch, Anne Preis, Peter Kötter, Otto Berninghausen, Thomas Becker, Roland Beckmann & Robert Tampé. 2017. Structure of the 40S–ABCE1 post-splitting complex in ribosome recycling and translation initiation. Nature Structural & Molecular Biology, published online 3 April 2017, doi:10.1038/nsmb.3396. Link…
Cluster of Excellence Macromolecular Complexes, Frankfurt am Main, Germany

